Abstract
Introduction For patients with high-risk acute leukemia (AL) lacking a matched sibling donor (MSD) or matched unrelated donor (MUD), an allogeneic stem cell transplant (alloSCT) from a haploidentical (HAPLO) donor is an increasingly used alternative. However, available data on the treatment of relapse after HAPLO-SCT is scarce. After a first transplant from MSD/MUD, a second allogeneic SCT (alloSCT2) using either MSD or MUD achieved 2-year overall survival (OS) in 20-30% of patients, independently of donor type for alloSCT2 and donor change. In contrast, there is little information supporting the feasibility and efficacy of a second HAPLO-SCT (HAPLO-SCT2) in patients with AL transplanted from a HAPLO donor at alloSCT1. On behalf of the Acute Leukaemia Working Party (ALWP) of the European Society of Blood and Marrow Transplantation (EBMT), we report here on the outcome of 82 patients with AL that underwent HAPLO-SCT2 after relapse from a first HAPLO- SCT in Europe.
Methods This was a registry-based analysis of adults with acute myeloid leukemia and acute lymphoblastic leukemia (AML/ALL). All consecutive patients that had received a HAPLO-SCT2 without ex-vivo manipulation after hematologic relapse from HAPLO-SCT1 between 2007 and 2021 were included. Outcomes of interest were engraftment, OS, leukemia-free survival (LFS), non-relapse mortality (NRM), relapse incidence (RI), graft-versus-host disease (GvHD), and GvHD-free, relapse-free survival (GFRS) rates after HAPLO-SCT2.
Results Eighty-two patients (AML, n=63, 77%; ALL, n=19, 23%) were identified. The median age at HAPLO-SCT2 was 47.2 years (range, 18.3-69.3) for AML and 33.5 (range, 19.7-58.2) for ALL (p=0.002), median interval from HAPLO-SCT1 to relapse was 7.5 months. A donor change between HAPLO-SCT1 and 2 was chosen in 35 (62.5%) of AML and 17 (89.5%) of ALL patients (p=0.042). Cell source was peripheral blood/bone marrow (PB/BM) in 15/67 (18%/82%), respectively. At HAPLO-SCT2, 21 (33.3%) AML patients had a complete remission (CR) and 49 (59.8%) an active disease, whereas 12 (63.2%) ALL patients were in CR (p=0.02). Reduced intensity conditioning (RIC) was used for HAPLO-SCT2 in 57% of patients, and post-transplant cyclophosphamide (PTCy) was the most frequent GvHD prophylaxis (82%).
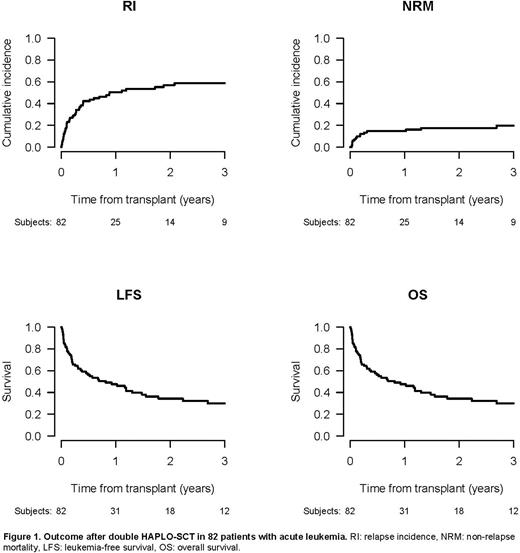
Following HAPLO-SCT2, median follow-up was 38/19 months for AML/ALL patients, respectively. In the entire cohort, 87% of patients engrafted. Cumulative incidences (CI) at day 180 of acute GvHD grade 2-4 and 3-4 were 23.9% and 15.3%, respectively. The CI of chronic GvHD and extensive chronic GvHD at 2 years was 22.6% and 11.2%, respectively. Overall, 2-year OS and LFS from HAPLO-SCT2 were 34.3% and 25.4%; 2-year NRM, RI, and GRFS rates were 17.6%, 57% 15.1%, respectively (Figure 1). When analysed separately by diagnose, 2-year OS/LFS were 28.7%/22.3% for AML and 55.3%/38.4% for ALL, respectively. NRM was relatively low (16.2%/23.5% in AML/ALL), while RI was considerable, reaching 61.6%/38.2% for AML/ALL, respectively. Consequently, leukemia was the most frequent cause of death.
Among the 63 patients with AML, an analysis of risk factors for OS/LFS from HAPLO-SCT2 identified disease status at HAPLO-SCT1 (p=0.008/p=0.026), and at Haplo-SCT2 (p=0.047/p=0.043) as well as the interval from HAPLO-SCT1 to relapse (p=0.001/p=0.001) as most important variables. In contrast, conditioning intensity and change of donor did not make a significant difference. No clear risk factor was identified for NRM.
Conclusion This is the largest cohort of patients undergoing double HAPLO-SCT analysed so far. Within the limits of a registry-based analysis, the data show the feasibility of a second HAPLO-SCT after relapse from HAPLO-SCT1 with high engraftment rates and acceptable NRM. Outcome data as well as risk factors are comparable to results reported after alloSCT2 in a matched donor setting. Results were encouraging among patients with ALL (n=19, only 2 with Philadelphia chromosome positive [Ph+] disease), who were younger and more often received HAPLO-SCT2 in CR and from a new donor. HAPLO-SCT2 is a viable option for AL patients relapsing after a HAPLO-SCT1.
Disclosures
Filippini Velazquez:Abbvie: Research Funding; JAZZ Pharmaceuticals: Research Funding. Labopin:Jazz Pharmaceuticals: Honoraria. Angelucci:Sanofi: Speakers Bureau; Vertex: Honoraria, Other: Data monitoring committee; Roche: Consultancy; Gilead: Consultancy; Glaxo: Consultancy; Bluebird Bio: Consultancy; Menarini/Stemline: Consultancy; Celgene: Honoraria, Other: Data monitoring committee; Novartis: Honoraria; Vifopr: Honoraria, Other: Data monitoring committee. Galieni:abbvie: Membership on an entity's Board of Directors or advisory committees; takeda: Membership on an entity's Board of Directors or advisory committees. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Schmid:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Abbvie: Research Funding. Ciceri:Kite Pharma: Consultancy. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding; Novartis: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria; Adaptive Biotechnologies: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal